Thursday, 19 November 2015
Carbohydrates Summary Notes
Biological Molecules: Carbohydrates
Carbohydrates>
A group of molecules containing C, H and O.
For every C atom there are 2H atoms and an O atom.
They act as:
A source of energy, ie. glucose
A store of energy, ie. starch and glycogen
Structural units, ie. cellulose and chitin
Some carbohydrates are part of other molecules such as nucleic acids and glycolipids.
There are three main groups of carbohydrates: monosaccharides, disaccharides and polysaccharides.
Monosaccharides>
The simplest carbohydrates.
Particularly important in living things as a source of energy.
Suited as an energy store because of the large numbers of hydrogen bonds.
Soluble in water and insoluble in non-polar solvents.
POLAR=WHEN THE CHARGE IS NOT EVENLY DISTRIBUTED ACROSS THE PARTICLE.
Disaccharides>
2 Monomers, (monosaccharides), bond together to form disaccharides.
Sweet and soluble.
Maltose, sucrose and lactose are typical examples.
When they join, a condensation reaction occurs to form a glycosidic bond. Two hydroxyl (OH) groups line up next to each other, from which a water molecule is removed, leaving an oxygen atom acting as a link between the monosaccharide units.
Disaccharides are broken into their monomers by the addition of water, a hydrolysis reaction.
Polysaccharides>
Polymers made from many monosaccharides.
3 important examples include: starch, glycogen and cellulose.
Are polymers, high molecular weight.
Diversity of structure.
Branched or linear chains.
One or more monosaccharide type.
Major importance to plants.
Minor roles in animals.
Hetero/homopolysaccharides.
Glycosidic bonds can form when the 'reducing group' of one monosaccharide is joined by a condensation reaction.
When you polymerise alpha glucose you produce an alpha helix.
Due to H bonding, the polymer of alpha glucose spontaneously folds into an alpha helix.
The helix structure allows a lot of glucose to be stored in a small space, highly compact structure.
Beta glucose is linear.
 |
| Credits to http://andrewpover.co.uk/biology/ocr-as-biology-amylose-amylopectin-cellulose-and-glycogen-comparison-table/ |
Tuesday, 17 November 2015
Oscar Wilde's Testimony
"The love that dare not speak its name" in this century is such a great affection of an elder for a younger man as there was between David and Jonathan, such as Plato made the very basis of his philosophy, and such as you find in the sonnets of Michelangelo and Shakespeare. It is that deep, spiritual affection that is as pure as it is perfect. It dictates and pervades great works of art like those of Shakespeare and Michelangelo, and those two letters of mine, such as they are. It is in this century misunderstood, so much misunderstood that it may be described as the "Love that dare not speak its name," and on account of it I am placed where I am now. It is beautiful, it is fine, it is the noblest form of affection. There is nothing unnatural about it. It is intellectual, and it repeatedly exists between an elder and a younger man, when the elder man has intellect, and the younger man has all the joy, hope and glamour of life before him. That it should be so the world does not understand. The world mocks it and sometimes puts one in the pillory for it.
Proteins- Revision Notes
Proteins
Amino acids>
Monomers of all proteins, all have the same basic structure.
Peptide bond>
A bond formed when two amino acids are joined by a condensation reaction.
Proteins are polymers comprised of long chains of amino acids. The properties of proteins give them a variety of functions:
They form components of animals, ie. muscle tissue
Adopt specific shapes- enzymes, antibodies and some hormones.
Membranes have protein constituents that act as carriers and pores for active transport across the membrane and facilitated diffusion.
Both plants and animals need amino acids to make proteins. Animals can make some proteins, but must ingest others, (essential amino acids).
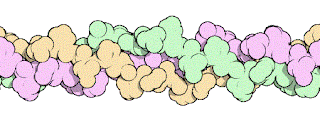 |
| Structure of Collagen |
Structure of an amino acid>
Each 'R Group' has a different and specific characteristic.
Cysteine has sulphur in its R Group so it forms strong covalent S-S disulphide bonds.
Peptide bonds join amino acids together.
2 amino acids join to form a dipeptide.
Primary Structure>
Peptide bonds formed between amine of one amino acid and combines with the OH carboxyl group of another amino acid.
This is a condensation reaction, where water is produced.
The peptide bond is covalent.
Secondary Structure>
Alpha helix or beta pleated sheet is formed from the primary structure due to the formation of hydrogen bonds between the amine group of one amino acid and the carboxyl group of another a short distance away.
Tertiary Structure>
Some R groups attract/repel.
Disulphide bridges/bonds form between cysteine/SH groups/S atoms.
Hydrogen/H bonds very important.
Ionic bonds also form between oppositely charged (+ and -) R groups and side chains.
Hydrophilic R groups arrange themselves on the outside of the protein molecule/in contact with water molecules whilst hydrophobic R groups are shielded from water molecules on the inside of the molecule.
Quaternary Structure>
The structure formed when two or more polypeptide chains join together, sometimes with an inorganic component, to form a protein.
 |
| Haemoglobin |
Monday, 16 November 2015
Feminist Perspectives on Education- Sociological Perspectives Notes and Statistics
Feminist Perspectives on Education- Sociology Notes
One of the main roles of education has been to maintain them gender inequalities.
Gendered language>
Textbooks and teachers tend to use gendered language, ie. him, his, he, when referring to person/people; assuming that non named subjects in research, articles are male.
This creates a male supremacy and makes women's role in education/research less encouraged or desirable.
Gendered roles>
Textbooks present men and women in traditional roles.
Women are presented as housewives and mothers whilst men are depicted as workers, (breadwinners).
Particularly evident in reading schemes during the 1960's/70's.
Gender Stereotypes>
Reading schemes present these.
Analysis of reading schemes from the 1960's/70's found that:
Boys are presented as more adventurous than girls.
Physically stronger than girls.
Having more choices than girls.
Girls presented as more caring/empathetic than boys.
More interested in domestic matters than boys.
Followers rather than leaders.
Women in the Curriculum>
Women have been 'hidden' from history- always been the history of men and in the perspectives of men. Does not create a unity that Functionalist sociologists emphasise history creates.
Subject choices-
Females avoid maths, science and technology.
Certain subjects seen as 'boys' and 'girls' subjects.
Often 'girls' subjects had lower status and market value.
Discrimination>
Against girls in ed. because of their gender.
Eg.- the 11+ in the 1940's, the pass mark for boys was lower so that boys and girls in grammar schools was roughly equal.
Girls were artificially 'failed' so boys could succeed.
Further and Higher Education>
The number of females that go on to higher education has been lower than girls.
Teachers gave more encouragement to boys to go to university than girls.
Evaluation>
Valuable for exposing gender inequality in ed., partly as a result of sociological research a lot has changed, eg. sexism in reading schemes has now disappeared.
Women have overtaken men on practically every measure of educational attainment-
The concern now is the underachievement of boys in ed., not the discrimination of girls.
Marxists criticise their disregard for class inequalities in causing educational differences between pupils.
Cell Membranes- Revision Notes
Cell Membranes
Cell membrane= the boundary between the cell and its environment, regulates what enters and exits the cell.
The cell membrane is composed of phospholipids, cholesterol, proteins and carbohydrates.
Phospholipids
Make up the basic structure of the cell; the phospholipid bilayer.
Phosphate head- polar, hydrophilic. Arranged so that they face intra and extracellular fluid.
Lipid tail- non-polar, hydrophobic.
Cholesterol
Hydrogen and carbon atoms.
Found among the phospholipid bilayer.
They keep the phospholipid tails from solidifying when temperature drops as it separates the tails to prevent crystallisation.
Keeps the membrane flexible.
Makes the membrane less soluble- prevents some water soluble molecules from passing through it.
Proteins
Integral proteins within the membrane, peripheral proteins outside of the bilayer.
Can act as enzymes to catalyse reactions, receptors of hormones etc or as transporters of materials across the cell membrane by facilitated diffusion.
Carbohydrates (Chains) and Glycoproteins
Glycoproteins form H bonds with water molecules surrounding the cell.
Stabilise the membrane structure.
Can act as antigens to help cells recognise one another.
Receptors of hormones or neurotransmitters.
Carbohydrate chains are attached to glycoproteins outside of the plasma membrane.
Cushions the membrane.
Important in cell recognition and can also act as a 'glue' to attach cells together.
Cell Membranes Website for Notes
Subscribe to:
Posts (Atom)












